The threat to flora and fauna within Australia and globally is not climatic change in itself, but the rapid pace at which anthropogenic Climate Change induced events are occurring, and the potential of positive feedback loops in abetting the sixth mass extinction in Earth’s history (Shavanna, 2020; Wagler, 2012; Wake & Vredenburg, 2008).
Natural variations in the earth’s climate system, driven by environmental forcings including Milankovitch cycles and volcanic activity, are continuously impacting plant and animal communities. However, the long-term time scales at which natural climatic events occur gives species ample time to adapt (Myhre et al. 2013).
Climatic change is complex
The myriad impacts a rapidly changing climate can have on wild plant and animal communities are far-reaching, therefore the following will focus on the impact of CO2 levels driving Climate Change and the consequent effect on the hydrologic cycle. Specifically, it will examine how rising global temperatures, ocean acidification and sea level rise can impact phenotypic traits and trophic relationships.
Around 3 billion years ago the evolution of photosynthetic organisms, which sequester carbon and produce oxygen (Des Marais, 2000; Ghommem, et al. 2012), initiated a chain of interrelated biological and geophysical events that changed the Earth’s atmospheric composition from ~80% carbon dioxide (CO2) to 78% Nitrogen and 21% Oxygen (NASA, 2020; NOAA, 2009; Rye et al. 1995).
Figure 1 The Complex Network of Climate System Processes
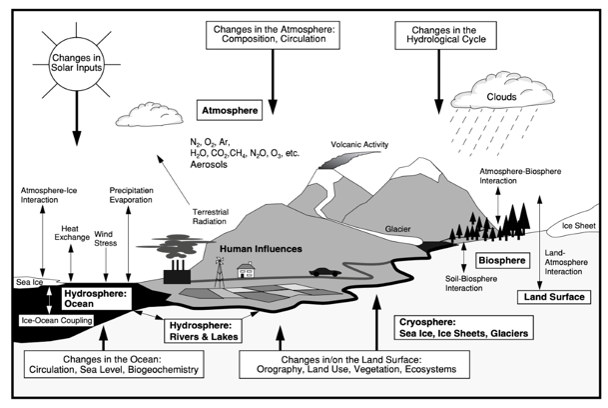
Over time, this created a balanced, cyclical climate system supported by a complex network of geophysiological processes, as shown in Figure 1. These climatic connections between the atmosphere, hydrosphere, lithosphere and biosphere, have successfully maintained a stable temperature range for hundreds of thousands of years, driving the evolution and diversification of plant and animal species on Earth (Beerling & Berner 2005; Glikson 2008; Shivanna 2020).
CO2 emissions are changing the climate
Recent changes in atmospheric CO2 levels are now threatening this once stable climate, and the plethora of living organisms that depend upon it for survival. In the past fifty years, anthropogenic emissions of CO2 have increased exponentially. As demonstrated in Figure 3, CO2 levels are currently higher than any period in the past 800,000 years (Scripps Oceanography, n.d).
This rise in atmospheric CO2 levels has resulted in an increase in global temperatures due to the ability of CO2 to absorb infrared radiation (Kikuchi 2008; Rye et al. 1995). This has offset the delicately balanced climate system by trapping an unsustainable amount of heat in the atmosphere, creating an enhanced greenhouse effect (IPCC 2013; IPCC 2014), more commonly known as Climate Change.
Figure 3 Increasing levels of atmospheric CO2 are higher than any time in the past 800,000 years

Climate Change impacts on Flora and Fauna
Increasing global temperatures influence phenotypic traits and impact trophic relationships of various plant and animal species reliant on specific climatic conditions to survive (Edwards & Richardson 2004; Hoegh-Guldberg et al. 2018; Jiapaer et al. 2015; Richards & Day 2018).
The effects have already begun to impact marine ecosystems, such as the iconic Great Barrier Reef, which despite being listed as a World Heritage Area, is suggested to be at risk of extinction in the near future (Richards & Day 2018).
The feminisation of Green Sea Turtles
Organism-environment interactions of egg-laying reptiles, with temperature dependent sex determination (TSD), such as the Green sea turtle (Chelonia mydas), are very sensitive to even a 1 ºC change in their thermal environment (Janzen, 1994). Listed in 2004 as endangered on the International Union for Conservation of Nature Red List (IUCN 2020), the Green sea turtle, shown in Figure 4, is showing signs of population feminisation, induced by Climate Change.
Figure 4 The Green Sea Turtle (Chelonia Mydas)

In the warm northern beaches of the Great Barrier Reef ecosystem, ecologists have found that 86.8% of adult sized turtles, 99.1% of juvenile turtles, and 99.8% of subadult turtles were born female, due to higher than usual nest temperatures (Jensen 2018).
In Taiwan, similar studies showed hotter temperatures and wetter conditions also produced female biased populations (King et al. 2013). Therefore, faunal communities with TSD around the world could be facing extinction as a result of rising temperatures.
Icons under threat from Climate Change
The Great Barrier Reef is not the only Australian icon feeling the heat from rising temperatures. The trophic relationship between the Eucalyptus population that currently dominates the Australian woodland regions (Carlos et al. 2016), and Australia’s famous koala population (Phascolarctos cinereus), is under threat.
Since being listed as vulnerable in 2014 on the IUCN Red list (IUCN 2020), the koala has been affected by deforestation and extreme weather events in its prime habitat of south-eastern Queensland and eastern New South Wales (Adams-Hosking et al 2011).
The 2019/2020 wildfires, scientifically correlated to Climate Change (McDonald, 2020), are estimated to have killed 70% of the population in fire-affected areas in Queensland (Biolink 2020), however the Climate Change effects threatening the koala do not stop there.
Co2 and the impact on plant tissue
Studies show increased atmospheric CO2 levels impact the chemical concentration and composition of foliar tissue (Beerling & Berner 2005; Faldyn et al 2018; Moore et al. 2004), amplifying the carbon:nitrogen ratio of plant species and potentially decreasing their nutritional value (Moore et al. 2004).
Figure 5 The Koala (Phascolarctos cinereus) in a Grey Gum (Eucalyptus punctata)

As koalas currently only consume 50 of the 700 species of Eucalypts in Australia (WWF 2018), one of which is the Grey Gum (Eucalyptus punctate) shown in Figure 5, a change in nutritional value to the koala’s primary food source could prove detrimental to an already vulnerable species.
According to Carlos et. Al (2016), phenotypic changes of Eucalypts as a response to Climate Change may not be the only cause for concern, with 2.4% of Australian eucalypt species predicted to become extinct, and 91% of the geographic range of endemic eucalypt species predicted to reduce by half within 60 years. If these predictions prove true, koalas will not only be affected trophically by rising CO2 levels, but their capacity to outpace Climate Change will be threatened further by a substantial decline in range and distribution.
The decline of the Monarch butterfly
Similar to the koala, the Monarch butterfly (Danaus plexippus) relies on a single foliar food source, Milkweed (Asclepias), however it may be at higher risk than the Australian folivore, due to a reduction in population size of 80% in the past two decades (Center for Biological Diversity 2020).
The Monarch is best known for its mass overwintering migration, however increasing temperatures have put both its migratory journey and primary food source at risk. When stressed by high temperatures, Monarch’s suffer from developmental issues and increased mortality rates (York & Oberhauser 2002) and unwittingly, this species of Rhopalocera is now also at risk of being caught in an ecological trap due to increasing CO2 levels (Battin 2004).
Caught in an ecological trap
An ecological trap occurs when a living organism responds to seemingly positive environmental cues, which have negative consequences. In the case of the Monarch, the ecological trap is the establishment of non-migratory populations, that carry an increased risk of disease, due to the improved performance of the invasive Milkweed species as temperatures increase, see Figure 6 (Faldyn et al., 2018).
Figure 6 Response of the invasive and native Milkweed species to rising temperatures
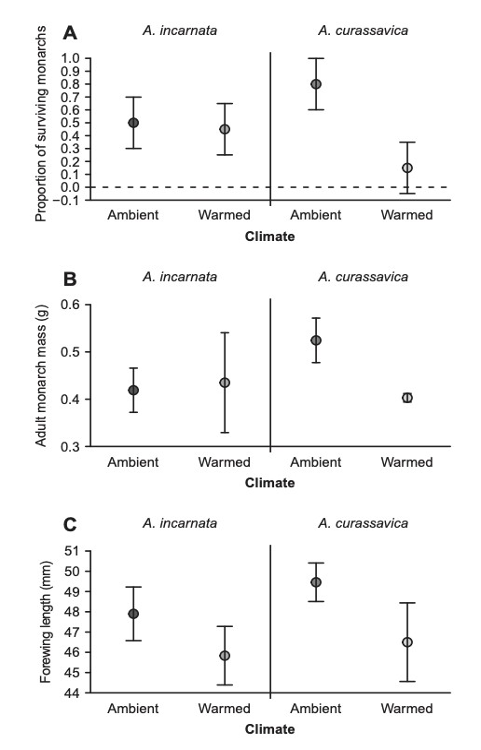
As is the case with many invasive plant species, the invasive Milkweed species (Asclepias Incarnata) is able to function at a higher capacity in warmer temperatures than the native Milkweed species (Asclepias Curassaciva), which has led to shifts in migration patterns (Lemoine, 2015).
With non-migratory Monarch populations now established in Central America, Australia, the United States, and the Pacific Islands (Nail et al. 2019) a further increase in temperatures could threaten the survival of migratory Monarch species, and other interconnected ecosystems that rely upon Monarchs to disperse seeds and pollinate plants on their migratory journey (Bascompte & Jordano 2007).
The role of water vapour in Climate Change
Increasing temperatures are driven by rising levels of CO2, however they are exacerbated by an increase of water vapour, and therefore interrelated hydrological processes and their effect on flora and fauna must be examined (Stagl et al. 2014).
Figure 7 Global Atmospheric Circulation Patterns
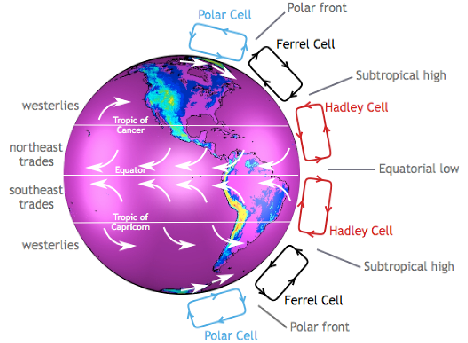
Water vapour is the most abundant gas in the atmosphere, responsible for transferring and storing energy via atmospheric circulation cells and ocean currents (see Figure 7 and Figure 8).
Figure 8 The Great Ocean Conveyor Belt

Water both affects and is affected by changes in global temperature. As temperatures rise, water changes from a liquid to a gas, creating latent heat in the process, increasing water vapour levels in the atmosphere. This increases humidity, further warms the earth, melts arctic ice, and in turn creates latent heat. This vicious cycle of warming, known as a positive feedback loop, is a key factor in understanding the cumulative effects of Climate Change and the consequences for flora and fauna.
Ocean acidification and Sea Level Rise
Ocean acidification and sea level rise are just two of the interrelated processes in the water cycle that threaten terrestrial and marine ecosystems.
Since the beginning of the industrial revolution 30% of human induced CO2 emissions (Sabine et al. 2004), have been absorbed by the earth’s oceans, triggering the process of ocean acidification. Ocean acidification poses a significant risk to marine organisms, as it can alter the phenotypic traits of calcifying marine invertebrates (Kelly & Hofmann 2013).
Figure 9 A closer look at the calcite shells of dinoflagellates
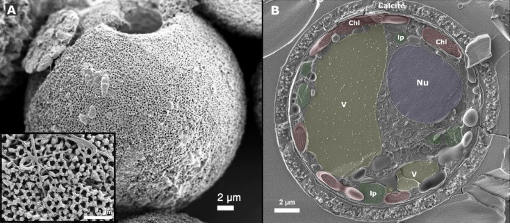
High levels of CO2 in the oceans increases the acidity of the water, which in turn impacts the solubility of CaCo3. This consequently impacts the calcification process of both single-celled organisms, including dinoflagellates as shown in Figure 9, and keystone bivalve organisms such as Laternula elliptica, shown in Figure 10 (Ji et al. 2016; McClintock et al. 2009; Van de Waal et al. 2013).
Figure 10 Laternula elliptica; a bivalve organism with a calcite shell
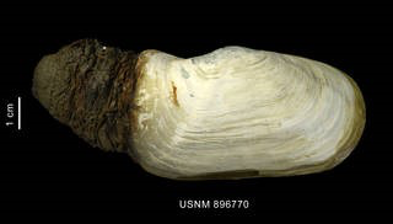
These marine invertebrates are reliant upon Calcium Carbonate (CaCO3) to form vital calcite structures, and in some cases are unable to complete their life cycles without them (The Royal Society 2005), suggesting that they may be at risk of extinction as CO2 levels continue to rise.
Coral bleaching and CO2
Ocean acidification has also been shown to pose a threat to the health of reef systems by means of coral bleaching, see Figure 11, which occurs when waters are between 30-34°C, and the symbiotic relationship between corals and zooxanthellae, a species of the dinoflagellates, is broken (Ralph et al. 2001).
Whilst studies on coral bleaching have predominantly focused on increasing sea surface temperatures, a study by Anthony et al. (2008) reveals a direct link between CO2 levels and coral bleaching, suggesting that Climate Change induced coral bleaching could be triggered by both increasing sea surface temperatures and ocean acidification.
Figure 11 Coral Bleaching on the Great Barrier Reef

Average sea surface temperatures are predicted to increase between 2.0 and 4.8°C by 2100 (Ficke et al. 2007), but warmer oceans are likely to trigger further coral bleaching events. They also pose a threat to the trophic (food chain) relationships of various marine organisms.
The impact of changing temperatures on the marine food chain
As the upper layers of the ocean warm, the boundary between warm surface ocean waters and the cool, dense waters of the ocean, are suppressed. These boundaries are known as thermoclines, shown in Figure 12, and typically stretch from 200m to 1000m below the sea surface.
Figure 12 The thermocline – a boundary between warm surface and cool deep ocean water.
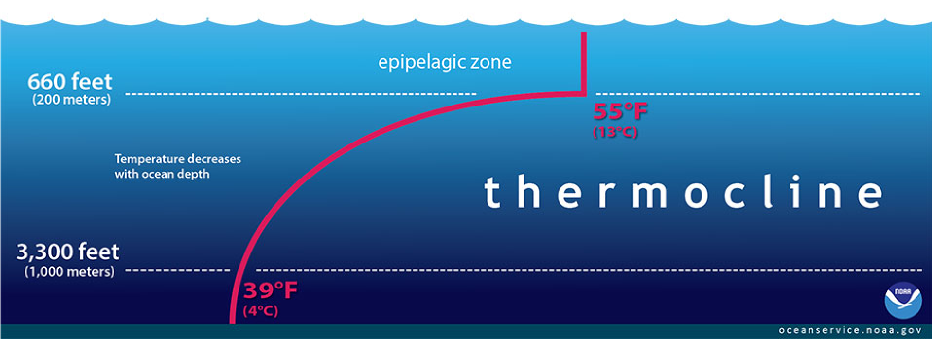
This has significant implications for relationships in ocean biomes and the marine food chain (Pelletier et al. 2012). A variety of marine species – both predators and prey – gather around thermoclines due to the nutrient rich water at the boundary line (Hansen et al. 2001; Pelletier et al. 2012).
Thermoclines; markers for food availability
The Australian little penguin (Eudyptula minor) and the Japanese seabird Rhinoceros Auklets (Cerorhinca monocerata) for example, are predators that rely on the thermocline for foraging success. In fact, the success of foraging above or near the thermocline has highlighted the potential for thermoclines to act as markers for enhanced food availability (Matsumoto et al. 2008; Pelletier et al. 2012).
If the oceans continue to warm, and thermoclines move further toward the ocean floor, predators may find it more difficult to hunt their prey. This is likely to impact nutrition, which can impair reproductive success, and disrupt the balance of the local ecosystem. Further, if the predator is migratory, a change in hunting conditions could threaten numerous other plant and animal communities along their migratory route.
The climate system is a complex network of interrelated processes and relationships, and this is just another example of how the effects of Climate Change on one species, can have farreaching consequences, as is the case with increasing sea surface temperatures and sea level rise.
The melting cryosphere and sea level rise
As sea surface temperatures increase, the ocean experiences thermal expansion, which along with the melting of land and sea ice (the cryosphere), is the key cause of rising sea levels.
Sea level rise, which is predicted to reach 0.61 – 1.10m above current levels by the end of the 21st century (IPCC 2020), has significant impacts upon not only the habitat, range and distribution of flora and fauna, but also on the salinisation of ground and soil water.
The plant and animal communities that will be most affected by sea level rise and salinisation are populations on low-lying islands and coastal areas. In fact a multitude of studies already predict that north-eastern New South Wales could lose up to 168.04 km2 of coastal wetlands and inland freshwater marshes by 2100 (Akumu et al. 2011).
The first mammalian extinction due to Climate Change
The first extinction of a mammal, that has been attributed to seal level rise as a result of Climate Change has already occurred (National Geographic 2019). The Bramble Cay Melomys (Melomys rubicola), as shown in Figure 13, was a species endemic to the low-lying island of Bramble Cay, in the eastern Torres Strait.
Figure 13 The first mammalian extinction from Climate Change induced sea level rise – Bramble Cay Melomys

Reported as extinct in 2016, the Bramble Cay Melomys was reliant on the endemic vegetation of the cay as its primary food source, and therefore the reduction of plant cover from 2.16 ha in 2004 to 0.065 ha in March 2014 (Watson 2016) impacted a vital trophic relationship.
The destruction of the Bramble Cay Melomys’ food source, alongside the repeated ocean inundation from sea level rise and resulting destruction of its only known habitat, suggest that whilst this species is the first recorded mammalian extinction accredited to sea level rise and Climate Change, it is unlikely to be the last (Waller et al. 2017).
The impact of groundwater salinisation in Florida
Sea level rise is also threatening pine forest communities in the Sugarloaf Key, an island in Florida Keys in the Gulf of Mexico, by increasing the salinisation of ground and soil water (Ross et al. 1994).
The issue with increasing salinity in ground water and soil is that salt content impacts plants’ ability to absorb water at the root system, which places stress on the photosynthetic process, and therefore its ability to absorb carbon dioxide. As with many other hydrologic processes, sea level rise as a result of Climate Change has cumulative effects on flora and fauna that will continue to exacerbate positive feedback loops and increase global warming.
The power of positive feedback loops
CO2 levels are currently at 411ppm, the highest level in human history (Scripps Oceanography, n.d), which is causing numerous impacts on the trophic relationships and phenotypic traits of wild plant and animal communities within Australia and globally.
Climate Change threats to flora and fauna vary in magnitude, from feminisation of egglaying reptiles to the first mammalian extinction as a result of Climate Change making the potential impacts of a rapidly changing climate unprecedented in scope.
Whilst anthropogenic CO2 emissions are responsible for the rapid pace at which temperatures are rising, the positive feedback loops of the hydrologic cycle, the most important positive feedback in the earth’s climate system (Stagl et al. 2014) are a key aspect in determining whether Climate Change is in fact abetting the sixth mass extinction in the earth’s history.
References
Australian Institute of Marine Science [AIMS]. (n.d). Coral Bleaching Events. Retrieved August 28, 2020 from https://www.aims.gov.au/docs/research/climate-change/coral-bleaching/bleaching-events.html
Adams-Hosking, C., Granthan, H.S., Rhodes, J.R., McApline, C., & Moss, P.T. (2011). Modelling climate-change-induced shifts in the distribution of the koala. Wildlife Research, 38. 122-130
Akumu, C., Pathirana, E., Baban, S., & Bucher, S. (2011). Examining the potential impacts of sea level rise on coastal wetlands in north-eastern NSW, Australia. Journal of Coastal Conservation, 15(1), 15–22. https://doi.org/10.1007/s11852-010-0114-3
Bascompte, J., & Jordano, P., (2007). Plant-animal mutualistic networks: the architecture of biodiversity. Annual Review of Ecology, Evolution and Systematics, 38. 567-593. https://doi.org/10.1146/annurev.ecolsys.38.091206.095818
Beerling, D.J., & Berner R. A. (2005). Feedbacks and the coevolution of plants and atmospheric CO2. PNAS, 102(5), 1302-1305. https://doi.org/10.1073/pnas.0408724102
Carlos E. González-Orozco, Laura j. Pollock, Andrew h. Thornhill, Brent d. Mishler, Nunzio Knerr, Shawn w. Laffan, Joseph t. Miller, Dan f. Rosauer, Daniel p. Faith, David a. Nipperess, Heini Kujala, Simon Linke, Nathalie Butt, Carsten Külheim, Michael d. Crisp, & Bernd Gruber. (2016). Phylogenetic approaches reveal biodiversity threats under climate change. Nature Climate Change, 6(12), 1110–1114. https://doi.org/10.1038/nclimate3126
Des Marais, D. J. (2000). Evolution: When did photosynthesis emerge on Earth? Science, 289, 1703–1705
Geographical. (2016). Extinction Watch: Bramble Cay Melomys. Retrieved August 28, 2020 from https://geographical.co.uk/nature/wildlife/item/1878-bramble-caymelomys
Ghommem, M., Muhammad R. H., & Ishwar K.P. (2012). Influence of natural and anthropogenic carbon dioxide sequestration on global warming. Ecological Modelling, 235-236. https://doi.org/10.1016/j.ecolmodel.2012.04.005
Glikson, A. Y. (2008) Milestones in the evolution of the atmosphere with reference to climate change, Australian Journal of Earth Sciences, 55(2), 125-139. https://doi.org/10.1080/08120090701689308
Edwards, M., & Richardson, A. (2004). Impact of climate change on marine pelagic phenology and trophic mismatch. Nature, 430(7002), 881-884. http://dx.doi.org.ezproxy.une.edu.au/10.1038/nature02808
Faldyn, M. J., Hunter, M. D., & Elderd, B. D. (2018). Climate change and an invasive, tropical milkweed: an ecological trap for monarch butterflies. Ecology, 99(5), 1031–1038. https://doi.org/10.1002/ecy.2198
Baede,. A.P., Ahlonsou, E., Ding, Y. & Schimel, D. (2001). The climate system: an overview. In Bolin, B., Pollonais, S. (Eds.) TAR Climate Change 2001: The Scientific Basis. (pp.85-98). In Press
Oppenheimer, M., Glavovic, B. C., Hinkel, J., Van de Wal, R., Magnan, A. K., Abd-Elgawad, A., Cai, Rongshuo., Cifuentes-Jara, M., Deconto, R. M., Ghosh, T., Hay, J., Isla, F., Marzeion, B., Meyssignac, B. & Sebesvari, Z. (2019). Sea level rise and implications for low-lying islands, coasts and communities. In Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V, Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., Petzold, J., Rama, B., & Weyer, N.M. (Eds.) IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. In Press
Intergovernmental Panel on Climate Change [IPCC]. (2014). Climate change 2014 synthesis report. In Pachauri, R.K. & Meyer, L.A. (Eds.) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Press
Hoegh-Guldberg, O., Jacob, D., Taylor, M., Bindi, M., Brown, S., Camilloni, I., Diedhiou, A., Djalante, R., Ebi, K.L., Engelbrecht, F., Guiot, J., Hijioka, Y., Mehrotra, S., Payne, A., Seneviratne, S.I., Thomas, A., Warren, R., & Zhou G. (2018). Impacts of 1.5°c of global warming on natural and human systems. In Masson-Delmotte, V., P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J.B.R. Matthews, Y. Chen, X. Zhou, M.I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, & T. Waterfield (Eds.), Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. (pp. 175 – 311). In Press.
Jantschke A., Pinkas I., Schertel A., Addadi L., & Weiner S. (2020). Biomineralization pathways in calcifying dinoflagellates: Uptake, storage in MgCaP-rich bodies and formation of the shell. Acta Biomaterialia, 102, 427-439.
Janzen, F.J., (1994). Climate Change and temperature-dependent sex determination in reptiles. Population Biology.91(16), 7487-7490. https://doi.org/10.1073/pnas.91.16.7487
Jensen, M. P., Allen, C. D., Eguchi, T., Bell, I. P., Lacasella, E. L., Hilton, W. A., Hof, C. A. M., & Dutton, P. H. (2018). Environmental Warming and Feminization of One of the Largest Sea Turtle Populations in the World. Current Biology, 28(1), 154–159.e4. https://doi.org/10.1016/j.cub.2017.11.057
Ji, Y., Xu, Z., Zou, D., & Gao, K. (2016). Ecophysiological responses of marine macroalgae to climate change factors. Journal of Applied Phycology, 28(5), 2953–2967. https://doi.org/10.1007/s10811-016-0840-5
Jiapaer, G., Liang, S., Yi, Q., & Liu, J. (2015). Vegetation dynamics and responses to recent climate change in Xinjiang using leaf area index as an indicator. Ecological Indicators, 58, 64–76. https://doi.org/10.1016/j.ecolind.2015.05.036
Kelly, M. W., & Hofmann, G. E. (2013). Adaptation and the physiology of ocean acidification. Functional Ecology, 27(4), 980–990. https://doi.org/10.1111/j.1365-2435.2012.02061.x
Kikuchi, R. (2008). Reconsideration of Climate Change from the Viewpoints of Greenhouse Gas Types and Time Scale. Energy & Environment, 19(5), 691–705. https://doi.org/10.1260/095830508784815946
King, Rowena, Cheng, Wan-Hwa, Tseng, Cheng-Tsung, Chen, Hochang, & Cheng, I-Jiunn. (2013). Estimating the sex ratio of green sea turtles (Chelonia mydas) in Taiwan by the nest temperature and histological methods. Journal of Experimental Marine Biology and Ecology, 445.
Kopp, G., & Lean, J. L. (2011). A new, lower value of total solar irradiance: Evidence and climate significance. Geophysical Research Letters, 38(1), n/a–n/a. https://doi.org/10.1029/2010GL045777
Lemoine, N.P. (2015). Climate change may alter breeding ground distributions of eastern migratory monarchs (Danaus plexippus) via range expansion of Asclepias host plants. PloS One, 10(2). https://doi.org/10.1371/journal.pone.0118614
Matsumoto, K., Deguchi, T., Wada, A., Kato, A., Saitoh, S., & Watanuki, Y. (2008). Estimating foraging area of Rhinoceros Auklets by simultaneous sampling of water temperature profiles using bird-borne data-loggers. The Ornithological Society of Japan, 7. 37-46.
McClintock, J. B., Angus, R. A., Mcdonald, M. R., Amsler, C. D., Catledge, S. A., & Vohra, Y. K. (2009). Rapid dissolution of shells of weakly calcified Antarctic benthic macroorganisms indicates high vulnerability to ocean acidification. Antarctic Science, 21(5), 449–456. https://doi.org/10.1017/S0954102009990198
McDonald, M. (2020). After the fires? Climate change and security in Australia. Australian Journal of Political Science. https://doi.org/10.1080/10361146.2020.1776680
Moore, B.D., Wallis, I.R., Marsh, K.J., Foley, W.J. (2004). The role of nutrition in the conservation of the marsupial folivores of eucalypt forests. School of Botany & Zoology. https://doi.org/10.7882/FS.2004.031
Myhre, G., Shindell, D., Bréon, F.-M., Collins, W., Fuglestvedt J., Huang, J., Koch, D., Lamarque J.-F., Lee, D., Mendoza, B., Nakajima, T., Robock, A., Stephens, G., Takemura, T., & Zhang, H. (2013). Anthropogenic and natural radiative forcing. In T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y.
Xia, V. Bex & P.M. Midgley (Eds.), Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. (pp. 659-740). Cambridge University Press.
Nail, K. R., Drizd, L., & Voorhies, K. J. (2019). Butterflies Across the Globe: A Synthesis of the Current Status and Characteristics of Monarch (Danaus plexippus) Populations Worldwide. Frontiers in Ecology and Evolution, 7. https://doi.org/10.3389/fevo.2019.00362
National Aeronautics and Space Administration [NASA]. (2020). Earth Fact Sheet. Retrieved August 16, 2020 from https://go.nasa.gov/3gDX07T
National Oceanic and Atmospheric Administration [NOAA]. (2009). Basics of the Carbon Cycle and the Greenhouse Effect. Retrieved August 17, 2020 from https://www.esrl.noaa.gov/gmd/education/carbon_toolkit/basics.html
National Oceanic and Atmospheric Administration [NOAA]. (2019). Why does the ocean get colder at depth?Retrieved August 28, 2020 from https://oceanservice.noaa.gov/facts/coldocean.html
National Oceanic and Atmospheric Administration [NOAA]. (n.d). What is a thermocline? Retrieved August 28, 2020 from https://oceanservice.noaa.gov/facts/thermocline.html
Pelletier, L., Kato, A., Chiaradia, A., & Ropert-Coudert, Y. (2012). Can Thermoclines Be a Cue to Prey Distribution for Marine Top Predators? A Case Study with Little Penguins. PLoS One, 7(4). https://doi.org/10.1371/journal.pone.0031768
Ralph, P. J., Gademann, R., & Larkum, A. W. (2001). Zooxanthellae expelled from bleached corals at 338C are photosynthetically competent. Marine Ecology Progress Series, 220, 163-168.
Richards, Z.T, & Day, J.C. (2018). Biodiversity of the Great Barrier Reef- how adequately is it protected? PeerJ. http://dx.doi.org.ezproxy.une.edu.au/10.7717/peerj.4747
Ross, M. S., O’Brien, J. J., & Da Silveira Lobo Sternberg, L. (1994). Sea-Level Rise and the Reduction in Pine Forests in the Florida Keys. Ecological Applications, 4(1), 144–156. https://doi.org/10.2307/1942124
Rye R., Kuo, P.H., & Holland, H. D. (1995). Atmospheric carbon dioxide concentrations before 2.2 billion years ago. Nature, 378(6557), 603–605. https://doi.org/10.1038/378603a0
Sabine, C.L., Feely, R.A., Gruber, N., Key, R.M., Lee, K., Bullister, J.L., Wanninkhof, R., Wong, C.S., Wallace, D.W.R., Tilbrook, B., Millero, F.J., Peng, T.H., Kozyr, A., Ono, T. <& Rios, A.F. (2004) The oceanic sink for anthropogenic CO(2). Science, 305, 367-371.
Scripps Oceanography. (n.d). The Keeling Curve. Retrieved August 16, 2020 from https://scripps.ucsd.edu/programs/keelingcurve/
Shivanna, K. R. (2020). The Sixth Mass Extinction Crisis and its Impact on Biodiversity and Human Welfare. Resonance, 25(1), 93–109. https://doi.org/10.1007/s12045-019-0924-z
Stagl J., Mayr E., Koch H., Hattermann F. F., & Huang, S. (2014). Effects of Climate Change on the Hydrological Cycle in Central and Eastern Europe. In Rannow, S., & Neubert, M. (Eds.) Managing Protected Areas in Central and Eastern Europe Under Climate Change. Advances in Global Change Research, 58. https://doi.org/10.1007/978-94-007-7960-0_3
The Royal Society. (2005). Ocean acidification due to increasing atmospheric carbon dioxide.https://royalsociety.org/topics-policy/publications/2005/ocean-acidification/
Van de Waal, D. B., John, U., Ziveri, P., Reichart, G.-J., Hoins, M., Sluijs, A., & Rost, B. (2013). Ocean Acidification Reduces Growth and Calcification in a Marine Dinoflagellate. PLoS ONE, 8(6). https://doi.org/10.1371/journal.pone.0065987
Voiland, A. (2017, July 26). In case you missed it: the tropics are coming the tropics are coming! Retrieved August 17, 2020 from https://go.nasa.gov/31CKOzL
Wagler, R. (2012). The Sixth Great Mass Extinction. Science Scope (Washington, D.C.), 35(7), 48–55. https://doi.org/10.2505/3/ss12_035_07
Wake, D. B., & Vredenburg, V. T. (2008). Are We in the Midst of the Sixth Mass Extinction? A View from the World of Amphibians. Proceedings of the National Academy of Sciences of the United States of America, 105(Supplement 1), 11466–11473. https://doi.org/10.1073/pnas.0801921105
Waller, N. L., Gynther, I. C., Freeman, A. B., Lavery, T. H., & Leung, L. K.-P. (2017). The Bramble Cay melomys (Rodentia: Muridae): a first mammalian extinction caused by human-induced climate change? Wildlife Research, 44(1), 9–21. https://doi.org/10.1071/WR16157
Watson, J. (2016). Bring climate change back from the future. Nature, 534(7608), 437. https://doi.org/10.1038/534437a
World Register of Marine Species. (n.d.). Laternula elliptica (P. P. King, 1832). Retrieved August 23, 2020 from http://www.marinespecies.org/aphia.php?p=taxdetails&id=197217#sources
World Wildlife Fund [WWF]. (2018). 10 Interesting facts about koalas. Retrieved August 21, 2020 from https://www.wwf.org.au/news/blogs/10-interesting-facts-about-koalas#gs.epkj1d
World Wildlife Fund [WWF] (2020). Green Turtle: Facts. Retrieved August 21, 2020 from https://www.worldwildlife.org/species/green-turtle
World Wildlife Fund [WWF]. (n.d). A review of the conservation status of koalas in QLD. Retrieved August 16th, 2020 from https://www.wwf.org.au/news/news/2020/following-bushfires-call-to-list-koalasas-endangered-in-qld-nsw-act#gs.eswqer
York H.A., Oberhauser K.S. (2002). Effects of Duration and Timing of Heat Stress on Monarch Butterfly (Danaus plexippus) (Lepidoptera: Nymphalidae) Development. Journal of the Kansas Entomological Society, 75(4), 290-298.

Leave a Reply